Biological membranes are hardly permeable to most water-soluble substances and are particularly difficult to cross for charged species. At the same time, many drugs are water soluble and have negatively charged functional groups in their structures. This slows down their penetration into cells, which may, in turn, weaken their therapeutic effect, e.g. antibacterial and anticancer activity. Synthetic anion transporters – small, drug-like organic molecules that facilitate the penetration of anions through lipophilic barriers – could help to solve this problem by accelerating the migration of anionic drugs across lipophilic membranes. Moreover, such transporters may be controlled by various stimuli, such as light, enzymes, or a change in pH, and thus could enable targeted delivery of drugs to the right place and at the right time.
Within this new grant, we aim to develop stimuli-responsive molecular transporters for anionic drugs. Such molecules could be useful, among others, in a fight against drug-resistant bacteria and cancer, some of the most serious contemporary health threats. Moreover, they might help to market drug candidates which suffer from low bioavailability, to limit side effects of particularly toxic drugs and to fight drug resistance.
If you like these ideas, join us in this research endeavour! We invite prospective MSc and PhD students as well as post-doctoral researchers to contact Prof. Michał Chmielewski at: mchmielewski@chem.uw.edu.pl
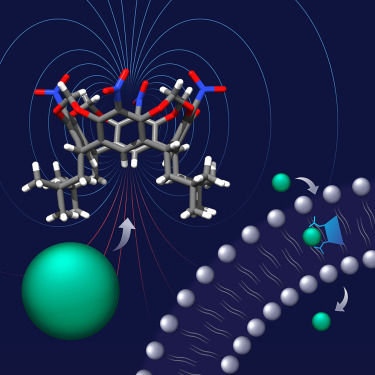
In collaboration with Professor Agnieszka Szumna from the Institute of Organic Chemistry, Polish Academy of Sciences, we show that resorcin[4]arenes, traditionally recognized as cation receptors, can be converted into powerful anion receptors and transporters through simple synthetic modification. The modified resorcin[4]arenes utilize CH—anion hydrogen bonds — typically considered as weak — for strong anion binding. This is possible thanks to their unique geometric features, which allow them to develop large dipole moments (up to 15.8 D) and concentrate highly positive electrostatic potential at the lower rim.
Furthermore, we show also that simple alkyl substitution around the anion binding site renders the receptors remarkably water tolerant and grants them outstanding anion transport properties. For example, the activity of 2 in Cl−/NO3− exchange across lipid bilayers surpasses the activity of the previously described CH-bonding transporters. Resorcin[4]arene 2 shows also remarkable Cl− > OH− selectivity in transport experiments, likely due to the soft nature of its CH donors and their resistance toward deprotonation. Such selectivity is highly desirable in medicinal applications because it is believed to minimize the toxicity of transporters.
Anion-templated synthesis is no longer limited to electrically charged catenanes and rotaxanes. Using doubly charged sulfate as a template and diamidocarbazoles as high affinity anion-binding building blocks, we obtained a fluorescent catenane with outstanding sulfate sensing capabilities. The catenane was shown ot bind sulfate very strongly, even in partially aqueous solutions. Moreover, we have shown that the sulfate anion may be considered as a pH-switchable template and hence it can be used to switch catenane between two significantly different states.
Have you ever struggled with low-yielding and non-reproducible post-synthetic modification of MOFs? Read our recent paper in MSDE for a possible solution!
Post-synthetic modification (PSM) is a powerful tool for introducing complex functionalities into metal–organic frameworks (MOFs). Aldehyde-tagged MOFs are particularly appealing platforms for covalent PSM due to the high reactivity of aldehyde groups, but the same feature also makes their solvothermal synthesis challenging. In this work, we show that while lowering the temperature during the synthesis of aldehyde-tagged UiO-68 avoids aldehyde group degradation and yields a highly porous and crystalline material, the resulting UiO-68–CHO contains a large fraction of missing linker defects and, as a result, its PSM is both inefficient and non-repeatable. However, we also show that this problem could be solved by 1) using an excess of linker during the synthesis of the MOF and 2) by soaking the crude material in the solution of the linker, which together reduce the density of defects enough to yield an excellent substrate for PSM. Treatment of the ‘healed’ material with model amines gives nearly quantitative conversions of aldehydes into imines, even if no excess of reagents is used. Importantly, the PSM of the ‘healed’ UiO-68–CHO gives repeatable results over many days, unlike the PSM of the highly defective MOF. Owing to these developments, various functionalities, such as new coordination sites, drug cargo, chirality, and hydrophobicity, were successfully introduced into the UiO-68 framework. The deleterious influence of defects on the PSM of MOFs and the solution to this problem proposed herein are likely to be of general nature, and hence might help in developing new and versatile platforms for covalent PSMs.
Congratulations to Krystyna Maslowska-Jarzyna on winning the START fellowship from the Foundation for Polish Science! Krystyna was selected among top 100 young polish scientists as one of only 10 chemists and the only chemist from our Department. The recipients were selected in a multi-stage competition on the basis of the quality of their scientific achievements.
For more, see the Foundation for Polish Science page.
In this paper, we propose a new strategy for the development of synthetic amino acid transporters. Such molecules might be expected to display a wide range of biological activities and also find applications in drug delivery, metabolism regulation, and as next-generation antibiotics.
The transport of amino acids across biological membranes is vital for the proper functioning of every living cell. This is because at physiological pH amino acids are very polar (they have a positively charged N-terminus and a negatively charged C-terminus) and therefore cannot pass through lipid bilayers alone. In nature, amino acid transport is carried out by specialized membrane proteins that play important roles in regulating key physiological functions, such as protein biosynthesis, metabolism, gene expression, redox balance and signaling. The dysfunction of these proteins contributes to the development of serious diseases, such as diabetes, neurodegenerative disorders, obesity and cancer. Synthetic amino acid transporters could help in treating these diseases, and might also find applications in drug delivery, metabolism regulation, and as next-generation antibiotics.
Unfortunately, precisely because amino acids are both cations and anions, the development of such synthetic amino acid transporters has so far been extremely difficult, since it required combining both cation and anion binding sites in one structure. In this paper, however, we show that this is not the only possible strategy and that even very simple anion transporters are able to efficiently transport amino acids across lipid bilayers at physiological pH. To explain this unexpected effectiveness of simple anionophores, we developed a new assay for studying the transport of amino acids, that gave us insight into the mechanism of this phenomenon. As a result, we were able to propose a new strategy to search for synthetic amino acid transporters with improved properties and interesting biological activity. Read on here.
The study reveals two distinct HCO3‾ transport mechanisms by simple di(thio)amidocarbazoles as well as their potent antimicrobial properties. Read more here.
Anions are typically too hydrophilic to freely pass through biological membranes. This also applies to those drugs that are anionic at physiological pH. Synthetic anion transporters, i.e. small, lipophilic molecules that facilitate diffusion of anions across lipophilic barriers, may accelerate the diffusion of anionic drugs by many orders of magnitude and thus could dramatically increase their effectiveness. Moreover, transporters whose activity could be controlled by light, pH or other stimuli could enable the targeted delivery of drugs to the desired place and at the right time. The aim of this project is to develop the first switchable transporters for anionic drugs, and hence to demonstrate a new strategy for targeted drug delivery. As part of it, we will undertake research on the construction of transporters switchable by changes in pH or irradiation with light of a specific wavelength. We hope that this research will lead to the development of a new strategy for smart drug delivery, which may find practical applications in the future, e.g. in the treatment of cancer.
A most warming welcome to our new post-doc: Dr. Debashis Mondal!
Debashis did his M.Sc. in chemistry from IIT Bombay (India) in 2015. He then enrolled at IISER Pune (India) for his doctoral studies in 2016, where he studied in the subfield of supramolecular chemistry under the supervision of Prof. Pinaki Talukdar. After completing his Ph.D. graduation in 2022, he joined the Supramolecular Chemistry group of Prof. Michał J. Chmielewski at the University of Warsaw as a post-doctoral fellow in September 2022.
Social media profile: https://twitter.com/DebashisJMChem
We are looking for 2 postdoctoral researchers for a groundbreaking research project on the border of organic, medicinal, and supramolecular chemistry.
The aim of the project is to develop small organic molecules capable of selectively transporting biologically relevant anions through lipid bilayers. Such molecules may exhibit interesting anti-cancer, antibacterial and antiviral properties, and may also find applications in the treatment of numerous diseases resulting from the dysfunction of natural transporters. Within the project, we would also like to construct stimuli-responsive transporters whose activity could be controlled by pH, light, or redox potential.
Successful candidates will design and synthesize novel anion receptors, study their anion binding properties and investigate their ability to transport anions through the lipid bilayers of model liposomes.
We offer:
Deadline for applications: 16 July 2022. More details in the following Announcement.