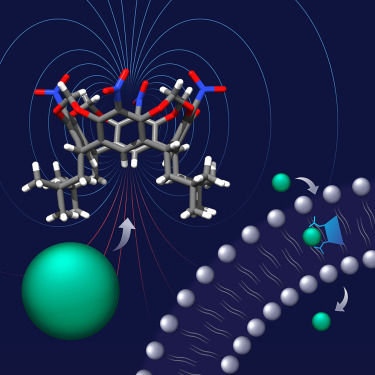
In collaboration with Professor Agnieszka Szumna from the Institute of Organic Chemistry, Polish Academy of Sciences, we show that resorcin[4]arenes, traditionally recognized as cation receptors, can be converted into powerful anion receptors and transporters through simple synthetic modification. The modified resorcin[4]arenes utilize CH—anion hydrogen bonds — typically considered as weak — for strong anion binding. This is possible thanks to their unique geometric features, which allow them to develop large dipole moments (up to 15.8 D) and concentrate highly positive electrostatic potential at the lower rim.
Furthermore, we show also that simple alkyl substitution around the anion binding site renders the receptors remarkably water tolerant and grants them outstanding anion transport properties. For example, the activity of 2 in Cl−/NO3− exchange across lipid bilayers surpasses the activity of the previously described CH-bonding transporters. Resorcin[4]arene 2 shows also remarkable Cl− > OH− selectivity in transport experiments, likely due to the soft nature of its CH donors and their resistance toward deprotonation. Such selectivity is highly desirable in medicinal applications because it is believed to minimize the toxicity of transporters.